Elblag, 25.01.2019
CERTIFICATE OF QUALITY
No 2019/026/2
Translation of CoQ No 2019/026/2
1.PRODUCT DESCRIPTION
|
Product trade name |
MCT oil with hemp extract |
|
Lot number |
325001 |
|
Production date |
15.01.19 |
|
Producer |
|
|
Country of origin |
Poland |
|
Ingredients and product description |
MCT oil with hemp extract with naturally occurring phytocannabinoids. Ingredients: MCT oil, CO2whole plant hemp extract, orange extract. |
|
Minimum durability date |
XI 2020 |
2. REQUIREMENTS
|
Parameter |
Limit |
Result |
|
Organoleptic characteristics |
The colour varies from yellow to yellow-brown. |
Meets requirements |
|
CBD content |
≥ 5 % (w/w) |
5.1 % (w/w) |
|
THC* content |
< 0.20 % (w/w) |
Meets requirements |
* total content of THC as a sum of Δ9–THC and THCA calculated with the following formula: THC = Δ9–THC +0,877 THCA
![]()
![]()
Prepared by –Anna Borawska, Eng. Accepted by– Paulina Wiśniewska, PhD,
Laboratory Technician Head of Laboratory

Certificate ID: 43765
Client Sample ID: Bath Bomb 4-1
Lot Number: 4
Received: 11/26/18
Scan QR Code
for authenticity

Tortuga life LLC 6241
Pembroke Rd
Hollywood, FL 33023
Matrix: Edibles -Baked Goods
Attn: SivanGolan
-
Authorization:
Rebecca Stevens, Chemist II
Signature: 
Date:
12/19/2018


 The data contained withing this report was collected in accodrdance with the requirements of ISO/IEC 17025:2005. I attest that the information contained within the report has been reviewed for accuracy and checked against the quality control requirements for each method. These results relate only to the test article listed in this report. Reports may not be reproduced except in their entirety.
The data contained withing this report was collected in accodrdance with the requirements of ISO/IEC 17025:2005. I attest that the information contained within the report has been reviewed for accuracy and checked against the quality control requirements for each method. These results relate only to the test article listed in this report. Reports may not be reproduced except in their entirety.
CN: Cannabinoid Profile &Potency[WI-10-17] Analyst:LG Test Date:12/14/2018
The client sample was analyzed for plant-based cannabinoids by Liquid Chromatography (LC). The collected data was compared to data collected for certified reference standards at known concentrations.
43765-CN
| ID | Weight% | Conc. | |||
|---|---|---|---|---|---|
| D9-THC | ND | ND | |||
| CBD | 0.03 wt % | 40.16 mg/bomb | 40 | ||
| CBDV | ND | ND | |||
| CBG | ND | ND | |||
| CBC | ND | ND | |||
| CBN | ND | ND | |||
| THCA | ND | ND | |||
| CBDA | ND | ND | |||
| CBGA | ND | ND | |||
| Total | 0.03wt% | 40.16mg/bomb | 0% | Cannabinoids(wt%) | 0% |
| Max THC | – | – | |||
| Max CBD | 0.03wt% | 40.16mg/bomb |
Max THC (and Max CBD) are calculated values for total cannabinoids after heating, assuming complete decarboxylation of the acid to the neutral form. It is calculated based on the weight loss of the acid group during decarboxylation: Max THC = (0.877 x THCA) + THC. ND = None detected above the limits of detection (LLD)
FM-10-05, Rev. 1, DCN:14-0001
420 Fortune Blvd • Milford, MA 01757 • 617-221-3356
www.ProVerdeLabs.com

CERTIFICATE OF ANALYSIS Cert No: C-AR0211-5-1
Sample information
Customer: George Botanicals
Sample type: CBD Balm, 300mg
Sample batch number: b-3369-2
PV sample ID: AR0211-5
Sample received date: 24.01.19
Method Information
Instrument ID: PVQ001
Method ID: PV001 Column ID: PVC002
Data sequence file:
ANALYSIS-2019-02-01 12-15-39
CANNABINOID PROFILE
|
Analyte |
%w/w |
|
CBDV |
0.21 |
|
CBDVA |
<0.05 |
|
CBD |
1.04 |
|
THCV |
0.08 |
|
CBDA |
<0.05 |
|
CBGA |
<0.05 |
|
CBN |
<0.05 |
|
Δ9-THC |
<0.05 |
|
Δ8-THC |
<0.05 |
|
THCVA |
<0.05 |
|
CBC |
<0.05 |
|
THCA |
<0.05 |
|
CBCA |
<0.05 |
- 0%
- 21%
- 0%
- 0%
- 100%
- 8%
CBC = Cannabichromene
CBD = Cannabidiol
CBDA=Cannabidiolicacid
CBDV=Cannabidivarin
CBDVA=Cannabidivarinicacid
CBG = Cannabigerol
CBGA=Cannabigerolicacid
CBN =Cannabinol
CBCA=Cannabachromenicacid
Δ9-THC =Δ9-tetrahydrocannabinol
Δ9-THCA =Δ9-tetrahydrocannabinolic acid
THCV = Tetrahyrdocannabivarin
THCVA = Tetrahydrocannabivarinic acid
Δ8-THC =Δ8-tetrahydrocannabinol
The test results relate only to the sample/
ssentin by the submitter.By placing the order for services with PhytoVistaLaboratories,terms and conditions are deemed to be accepted by the submitter.

Use the QR code to check
your results and find out
more information on how to


SAMPLE CONTROL d.o.o. Puškarićeva ulica 18, 10250 Lučko, Croatia; t. +385 6288 637 f. +385 1 6288 637
Analytical report for sample: 8491/2019 Date report:16.05.2019
Sample:SOMNIO CBD – ANTI AGE MOISTURISER – 30ML/30G
INFORMATION OF SAMPLING:
Date and time of sampling:13.05.201913:30
Place:Notspecified
Sample code:17296
Sampled by:Customer
Date and time of receipt the sample:13.05.201914:00
SAMPLE DESCRIPTION:
The sample was delivered in its own packaging in sufficient quantity for analysis.
Date and time of the analysis:13.05.2019 14:15
Date and time of completion of the analysis:16.05.2019 09:22
RESULTS OF ANALYSIS
Search type:Physical and chemical properties
| Parameter | Method | Unit of measurement | Limits | Result |
|---|---|---|---|---|
| Cannabidiol (CBD) | In house method-RU-MET-212 UPLC-MS/MS | g/100g | - | 1,3 |
| Delta-9-tetrahydrocannabinol (THC) | In house method-RU-MET-212 UPLC-MS/MS | g/100g | - | 0,0023 |
* refers to the method that has been accredited. Further information about the used methods can be obtained in the laboratory.
Analyst:
Vladimir Stankov, M.Sc.
![]()
Head of laboratory:
Anamarija Ilijaš, M.Sc. in exchange
![]()
This analytical report refers only to the submitted sample.
The analytical report is the result of electronic data processing, and the booklet, without stamp and signature.
End of analytical report.
![]()
Samplecontrol d.o.o. is accredited to the requirements of HRNENISO/IEC17025:2007 by the Croatian Accreditaon Agency for the field of food,feed,water,cosmetics and microbiological purity facilities and sampling of water for human consumption.The accreditation are a is specified in theAccreditation Certificateno.1257.
Analytical report for sample: 8491 / 2019
The test report must not be copied or reproduced without the written permission of the laboratory.

SAMPLE CONTROL d.o.o. Puškarićeva ulica 18, 10250 Lučko, Croatia; t. +385 6288 637 f. +385 1 6288 637
Analytical report for sample: 8491/2019 Date report:16.05.2019
Sample:SOMNIO CBD – ACNE SERUM – 30L/27G
INFORMATION OF SAMPLING:
Date and time of sampling:13.05.20191 3:30
Place:Not specified
Sample code:17294
Sampled by:Customer
Date and time of receipt the sample:13.05.2019 14:00
SAMPLE DESCRIPTION:
The sample was delivered in its own packaging in sufficient quantity for analysis.
Date and time of the analysis:13.05.2019 14:15
Date and time of completion of the analysis:16.05.2019 09:22
RESULTS OF ANALYSIS
Search type:Physical and chemical properties
| Parameter | Method | Unit of measurement | Limits | Result |
|---|---|---|---|---|
| Cannabidiol (CBD) | In house method-RU-MET-212 UPLC-MS/MS | g/100g | - | 1,27 |
| Delta-9-tetrahydrocannabinol (THC) | In house method-RU-MET-212 UPLC-MS/MS | g/100g | - | 0,0046 |
* refers to the method that has been accredited. Further information about the used methods can be obtained in the laboratory.
Analyst:
Vladimir Stankov, M.Sc.
![]()
Head of laboratory:
Anamarija Ilijaš, M.Sc. in exchange
![]()
This analytical report refers only to the submitted sample.
The analytical report is the result of electronic data processing, and the booklet, without stamp and signature.
End of analytical report.
![]()
Samplecontrol d.o.o. is accredited to the requirements of HRNENISO/IEC17025:2007 by the Croatian Accreditaon Agency for the field of food,feed,water,cosmetics and microbiological purity facilities and sampling of water for human consumption.The accreditation are a is specified in theAccreditation Certificateno.1257.
Analytical report for sample: 8491 / 2019
The test report must not be copied or reproduced without the written permission of the laboratory.
Elbląg, 25.01.19
CERTIFICATE OF ANALYSIS no2019/024/01
Translation of CoA no 2019/024/01
|
Sample description |
||||
|
MCT oil with CO₂hempextract (mint) |
||||
|
Sampling date |
Analysis date |
Sample collected |
||
|
15.01.19 |
16.01.19 |
|||
|
Sample name |
Laboratory code |
|||
|
324001 |
324001 |
|||
|
Remarks |
– |
|||
Results of analysis:
|
No |
Code |
Compound |
Analysis method |
Result |
Uncertainty |
Unit |
|
Cannabinoid content: |
||||||
|
1 |
324001 |
CBD |
HPLC (internal monograph) |
5.2 |
0.2 |
% (w:w) |
|
2 |
CBDV |
0.055 |
0.005 |
% (w:w) |
||
|
3 |
CBDA |
0.052 |
0.003 |
% (w:w) |
||
|
4 |
CBG |
0.21 |
0.01 |
% (w:w) |
||
|
5 |
CBN |
< 0.01 |
— |
% (w:w) |
||
|
6 |
CBC |
0.30 |
0.02 |
% (w:w) |
||
|
Terpenoid content: |
||||||
|
1 |
324001 |
BCP |
HPLC (internal monograph) |
0.06 |
0.01 |
% (w:w) |
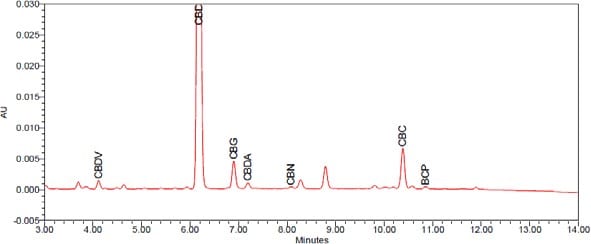
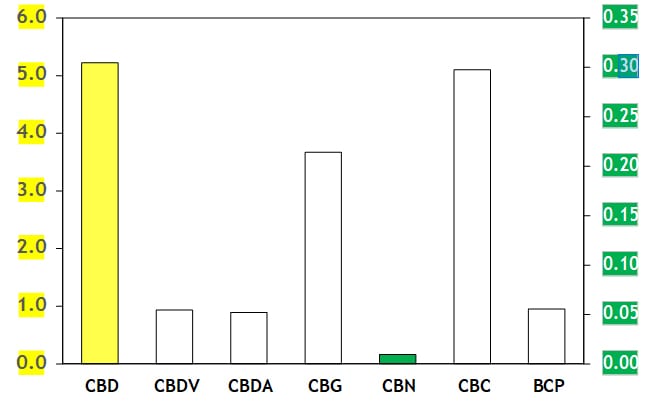
Cannabinoid and terpenoid percentages:
List of substances:
|
Substance name |
Symbol |
Structure |
Substance name |
Symbol |
Structure |
|
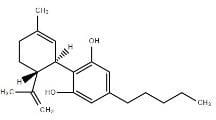
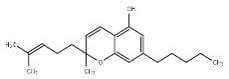
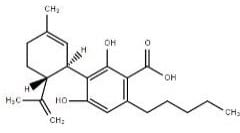
Cannabidiol |
CBD |
|
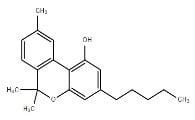
Cannabinol |
CBN |
|
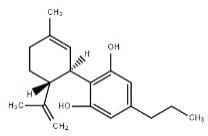
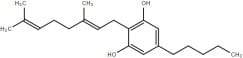
|
Cannabidivarin |
CBDV |
|
Cannabichromene |
CBC |
|
|
Cannabidiolic acid |
CBDA |
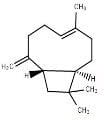
|
β-caryophyllene |
BCP |
|
|
Cannabigerol |
CBG |
|
|||
![]()
(Prepared by – Anna Borawska, Eng.,
Laboratory Technician)
![]()
(Accepted by – Paulina Wiśniewska, PhD.,
Head of Laboratory)
Measurement uncertainty estimated as the expanded uncertainty with a coverage factor k=2. corresponding to a level of confidence of about 95%.
Mesuerement uncertainty does not include sampling contribution.
This certificate shall not be reproduced expect in its entirety. without the written approval of the laboratory. Results are applicable only for samples tested.
Complaints should be submitted within 14 days from the date of certification.